
A Reversible Aluminium Metal Anode Enabled by Amorphisation in Aqueous Aluminium Batteries
Synopsis
Introducing an amorphous aluminium interfacial layer for aqueous aluminium metal batteries could resolve challenges of ineffective plating and parasitic reactions. This advancement enables a notable increase in discharge voltage while utilising a non-toxic, environmentally friendly electrolyte, paving the way for sustainable large-scale applications.
Opportunity
Aqueous aluminium ion batteries represent the next generation of post-lithium ion batteries, offering high safety and low cost. However, their development has been hindered by challenges such as ineffective aluminium plating in aqueous solutions and severe parasitic reactions—long-standing issues that are also prevalent in the metal refinery industry.
Amorphisation has been shown to be an effective strategy to tackle these critical issues. By thermodynamically shifting the reduction potential for aluminium deposition, this approach, previously unexplored in aluminium ion batteries, significantly enhances battery performance. The resulting batteries exhibit approximately a 0.6 V increase in discharge voltage plateau compared to bare aluminium-based cells, surpassing all previously reported aqueous aluminium ion batteries.
In addition, the current development enables the use of a non-corrosive, low-cost and fluorine-free Al2(SO4)3 electrolyte, which is eco-friendly and can be easily adapted for sustainable large-scale applications. In contrast, other reported aqueous aluminium ion batteries rely on expensive and toxic Al(OTF)3 electrolytes.
Technology
The invention relates to an amorphous aluminium (a-Al) interfacial layer as an anode for aqueous aluminium metal battery and a method of fabricating the same.
Aqueous aluminium metal batteries are plagued by ineffective aluminium plating in aqueous solutions and severe parasitic reactions, leading to limited discharge voltage.
The invention represents an amorphisation strategy to tackle these critical issues with the metal aluminium anode by thermodynamically shifting the reduction potential for aluminium deposition. The amorphous aluminium (a-Al) interfacial layer is triggered by in-situ lithium-ion alloying/de-alloying process on a low-strength metal aluminium substrate.
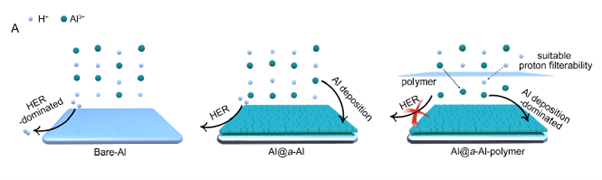 Figure 1: Amorphous aluminium anode for aqueous aluminium metal batteries
Figure 1: Amorphous aluminium anode for aqueous aluminium metal batteries
Applications & Advantages
- Aluminium's cost-effectiveness, high conductivity and charge storage capacity make it ideal as a battery anode.
- During charging, aluminium ions release from the anode and move through the electrolyte to the cathode. Upon discharge, these ions return to the anode, producing an electric current.
- Aluminium anodes enable rapid recharging as aluminium ions efficiently shuttle between the anode and cathode, a major advantage of aqueous aluminium batteries.














/enri-thumbnails/careeropportunities1f0caf1c-a12d-479c-be7c-3c04e085c617.tmb-mega-menu.jpg?Culture=en&sfvrsn=d7261e3b_1)

/cradle-thumbnails/research-capabilities1516d0ba63aa44f0b4ee77a8c05263b2.tmb-mega-menu.jpg?Culture=en&sfvrsn=1bc94f8_1)


.tmb-listing.jpg?Culture=en&sfvrsn=b5366f51_1)











