
A Greener Way to Make Fertiliser
Synopsis
This technology presents a greener method for urea production, utilising ambient processing conditions to reduce the carbon footprint. It involves an electrocatalytic approach, coupling nitrate with carbon dioxide on an indium hydroxide catalyst.
Opportunity
This invention offers a novel process for developing a catalyst to form urea, a critical nitrogen fertiliser. Urea, a colourless solid, is the most popular form of nitrogen fertiliser, with global annual production estimated at 200 million tons, valued at $70 billion. Traditionally, urea is produced via the Haber-Bosch/Bosch-Meiser process, a thermocatalytic method requiring high temperatures and pressures. This invention produces urea at around 473K (~200 degC) and 143kPa, significantly reducing the carbon footprint associated with its production. By directly coupling nitrate (NO3−) with carbon dioxide (CO2) on an indium hydroxide catalyst, this power-to-urea approach achieves highly selective urea electrosynthesis under ambient conditions.
Technology
This strategy represents a greener way to produce fertiliser. It involves a method of forming urea using an electrocatalyst and a novel mechanism for its formation. The process demonstrates excellent performance in urea synthesis, achieving high yields, faradaic efficiency and N/C urea-selectivity. It involves a novel mechanism that captures surface electrons via CO2, transforming semiconductor behaviour and suppressing HER. Further, the preferential coupling of *CO2 with *NO2 at an early stage ensures high selectivity in C-N bond formation.
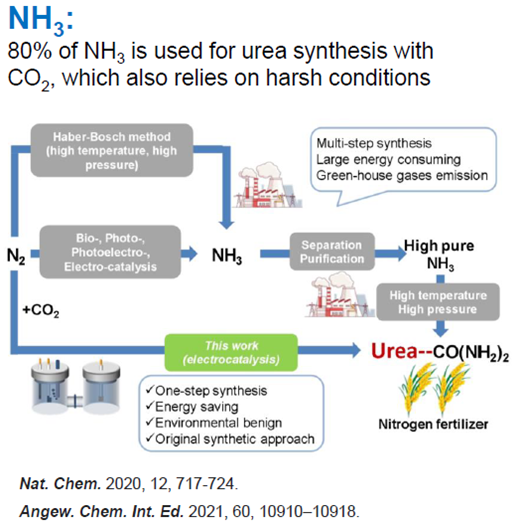
Figure 1: Process for developing a catalyst to form urea.
Applications & Advantages
Applications:
Sustainable Agriculture - Greener fertilisers support sustainable agriculture initiatives by minimising the ecological footprint of farming operations.
Aquaculture - These fertilisers promotes healthy aquatic plant growth and nutrient cycling in aquaculture systems.
Advantages:
- Minimises nutrient runoff and leaching, reducing water pollution and ecosystem degradation.
- Releases nutrients gradually, matching plant uptake patterns and reducing nutrient wastage.
- Reduces greenhouse gases emissions like nitrous oxide, a potent contributor to climate change.
- Enhances soil health by promoting beneficial microbial activity and increasing organic matter content.
- Contains fewer synthetic chemicals and residues, leading to healthier and safer food production.
- Supports soil structure and nutrient balance, preventing soil degradation and erosion.
- Biodegradable and breaks down naturally, reducing long-term environmental impacts.
- Supports biodiversity by reducing nutrient imbalances that favour certain plant species over others.


.tmb-listing.jpg?Culture=en&sfvrsn=ab6472c8_1)

.tmb-listing.jpg?Culture=en&sfvrsn=b5366f51_1)










