
A Method of Synthesising Atomically Dispersed CO2–N6 and Fe–N4 Costructures Anchored on Hierarchical Porous Carbon Host
Synopsis
This invention presents new ternary-atom catalysts, featuring Co–Co dimers and Fe single sites anchored on graphitised carbon. These catalysts outperform single-atom configurations for oxygen reduction due to synergistic effects between Co and Fe, enhancing *OH binding. This promising alternative to platinum (Pt) is highly effective in zinc-air batteries.
Opportunity
Rechargeable metal-air batteries, reliant on electrocatalysts for the oxygen reduction reaction (ORR), are emerging as next-generation renewable energy storage systems. Polynary transition-metal atom catalysts are poised to replace Pt-based catalysts for ORR. Regulating the local configuration of atomic catalysts is thus crucial for enhancing performance. This technology offers a novel approach to achieve this.
Technology
This invention introduces a precise synthesis strategy to design and construct atomically dispersed and nitrogen-coordinated ternary-atom centred sites anchored on CNTs-grafted hollow carbon. The unique atomic ORR catalyst outperforms those with only Co2-N6 or Fe-N4 sites in both alkaline and acidic conditions. Density functional theory calculations reveal the synergistic effect of Co2-N6 or Fe-N4 sites, which induce a higher filling degree of Fe-d orbitals and improve the binding capability to *OH intermediates. This ternary-atom catalyst is a promising alternative to Pt for driving cathodic ORR in zinc-air batteries.
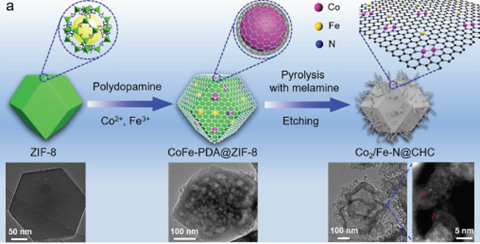
Figure 1: Schematic of the synthesis strategy of Co2/Fe–N@CHC and corresponding TEM images
Applications & Advantages
Main application areas include catalysts for ORR, zinc-air batteries and energy storage systems.
Advantages:
- Co2-N6 and Fe-N4 sites provide superior performance for ORR
- Exhibits higher power density in zinc-air batteries compared to Pt/C-based catalysts
- Demonstrates improved cycling stability compared to Pt/C-based catalysts



.tmb-listing.jpg?Culture=en&sfvrsn=b5366f51_1)











