Killing tumours with a light, airy touch
The destruction of tumour cells by light can be enhanced with nanoagents that generate oxygen in tissues, an NTU team finds.
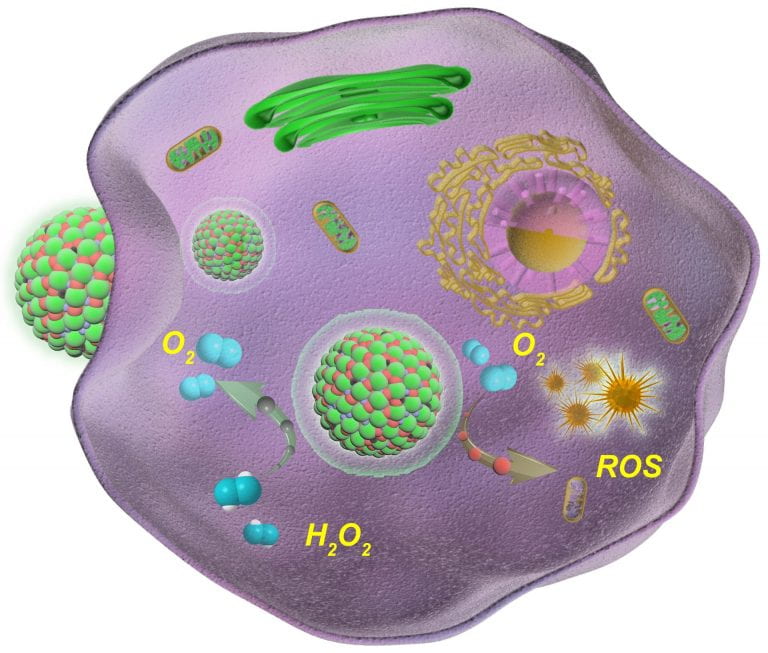
Schematic showing oxygen (O2) generation catalysed by an enzymatic nanoagent (green-red balls) to enhance the efficiency of photodynamic cancer therapy. Credit: Zhao Yanli/reproduced from Nature Communications (2020), DOI: 10.1038/s41467-019-14199-7.
Photodynamic therapy, which uses molecular oxygen to generate highly cytotoxic oxygen species that kill cancer cells, is an effective clinical treatment for superficial and localised tumours. It also has fewer side effects compared to conventional radiotherapy, chemotherapy and surgery.
The efficiency of photodynamic therapy is compromised, though, if oxygen levels are low in fast growing tumours. In addition, photodynamic therapy induces the collapse of tumour blood microvessels, causing further oxygen deprivation (hypoxia).
To break the vicious cycle of photodynamic therapy-induced tumour hypoxia, Prof Zhao Yanli of NTU’s School of Physical and Mathematical Sciences and his colleagues developed a self-assembled nanoagent that is able to generate oxygen inside tissues.
Serving a similar role as the enzyme catalase, the nanoagent raised local oxygen levels by catalysing a reaction between single-atom ruthenium nanoparticles and hydrogen peroxide present in tumour microenvironments.
A combination of the catalysis-based nanoplatform and photodynamic therapy resulted in the elimination of tumours in a mouse model, a result that could not be achieved by photodynamic therapy alone.
The nanoagent could also be used as a cancer theranostic to image and track tumours inside the body, the researchers say.
The research study “Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor” was published in Nature Communications (2020), DOI: 10.1038/s41467-019-14199-7.
The article appeared first in NTU’s research & innovation magazine Pushing Frontiers ( issue #17, August 2020).














/enri-thumbnails/careeropportunities1f0caf1c-a12d-479c-be7c-3c04e085c617.tmb-mega-menu.jpg?Culture=en&sfvrsn=d7261e3b_1)

/cradle-thumbnails/research-capabilities1516d0ba63aa44f0b4ee77a8c05263b2.tmb-mega-menu.jpg?Culture=en&sfvrsn=1bc94f8_1)

7e6fdc03-9018-4d08-9a98-8a21acbc37ba.tmb-mega-menu.jpg?Culture=en&sfvrsn=7deaf618_1)














.tmb-listing.jpg?Culture=en&sfvrsn=83fa0b1d_1)


-and-professor-hu-xiao-from-ntu-singapore.tmb-listing.jpg?Culture=en&sfvrsn=d0bcffec_1)
























