Kidneys in a dish
Nanyang Asst Prof Xia Yun grows miniature kidneys from stem cells to find therapies for kidney disease.

An inconspicuous pair of bean-shaped organs at the back of our abdomen, the kidneys perform crucial functions in organisms, including filtering blood, maintaining body fluid homeostasis and producing important hormones.
The global incidence of kidney failure is increasing at an alarming rate, in particular due to two major killer medical conditions of modern society— diabetes and cardiovascular disease.
According to the National Kidney Foundation Singapore, Singapore is ranked fourth worldwide for prevalence of kidney failure and first worldwide for diabetes-induced kidney failure. However, therapeutic approaches to tackle kidney disease— mainly dialysis and kidney transplants—have not changed fundamentally for many decades. Hence, there is a pressing need to develop novel approaches for better understanding and management of kidney disease.
Miniature kidneys in a petri dish
The last decade has seen global efforts in generating 3D mini organs (or organoids) from various cell types, including pluripotent stem cells, adult tissue stem cells and cells derived from tumour biopsies.
These organoids have provided unprecedented opportunities for us to gain a better understanding of human organ development, body homeostasis and pathogenesis. Most importantly, generating patient-derived miniature organs makes it possible to evaluate drug efficacy before clinical trials in a patient-specific manner.
Nevertheless, the current generation of mini organs is not ready for use in tissue replacement therapy due to limitations such as a lack of vasculature and proper organ maturation.
Next-generation organoids
To overcome current problems in kidney organoids, my lab set out to establish novel approaches for the differentiation of human pluripotent stem cells—cells that are potentially able to produce any cell or tissue in the body—into 3D kidney organoids.
Because the renal vascular network is critically associated with kidney function and defects, we aimed to devise kidney organoids that feature a vascular network. We developed a protocol to coax human pluripotent stem cells into kidney organoids with segmentally patterned nephrons—the kidney’s structural and functional units for blood filtration—and a highly elaborate vascular network (Figure 1).
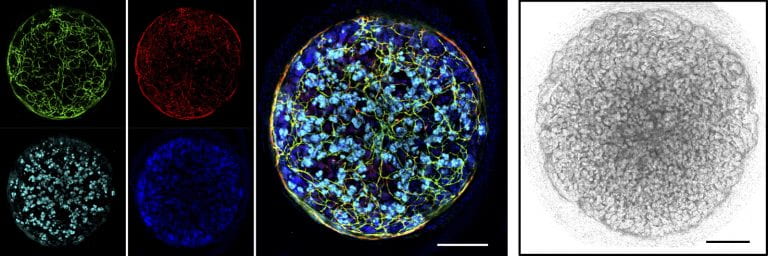 Figure 1: 3D kidney organoids at the 24-day growth stage (left) showing networks of blood vessels (vessel-lining endothelial cells are labelled in green and red); the kidney-specific protein nephrin and cell nuclei are labelled in light blue and dark blue, respectively; (right) light-microscopy image; scale bars: 200μm. Credit: Cell
Stem Cell (2019), DOI: 10.1016/j.stem.2019.06.009, reproduced with permission.
Figure 1: 3D kidney organoids at the 24-day growth stage (left) showing networks of blood vessels (vessel-lining endothelial cells are labelled in green and red); the kidney-specific protein nephrin and cell nuclei are labelled in light blue and dark blue, respectively; (right) light-microscopy image; scale bars: 200μm. Credit: Cell
Stem Cell (2019), DOI: 10.1016/j.stem.2019.06.009, reproduced with permission.
By changing the relative proportion of distinct nephron segments, we generated kidney organoids that are enriched in either glomeruli or tubules—two key structures of nephrons responsible for distinctive functions such as filtration, reabsorption, secretion and excretion.
When implanted into the kidney capsule of immune-compromised mice, the tailored organoids further matured and acquired the distinct capabilities of proximal and distal nephron segments in performing blood filtration and size-selective reabsorption of water and metabolites (Figure 2).

Figure 2: Kidney organoids with segmentally patterned nephron-like structures shown to be able to perform blood filtration and reabsorption of water and metabolites. Labelled structures: glomerulus (in blue), proximal, medial and distal tubules (in white, green and red, respectively). Credit: Cell Stem Cell (2019), DOI: 10.1016/j.stem.2019.06.009, reproduced with permission.
Despite these encouraging observations, our kidney organoids are far from being applicable for kidney replacement therapy. However, they are very useful for studying the many aspects of human renal biology that can’t be studied in either animal models or in vitro tissue cultures. Thus, using the organoids—alongside other tools such as single-cell RNA sequencing in a collaboration with Nanyang Asst Prof Foo Jia Nee—we were able to unveil a subset of nephron progenitor cells that can differentiate into renal vasculature, shedding light on a novel theory for kidney-specific vasculature formation.
We are currently developing an in vitro cell lineage tracing system to provide further evidence that human renal vasculature has multiple developmental origins.
Organoids as disease models
We are highly interested in using our kidney organoid platform to study genetic kidney diseases. To this end, we generated induced pluripotent stem cells (iPSC) derived from patients with inherited polycystic kidney disease, one of the most common genetic kidney disorders. With the help of CRISPRCas9 gene editing, we were able to change the cyst-developing phenotype in kidney organoids grown from these cells to a phenotype resembling healthy kidneys (Figure 3).
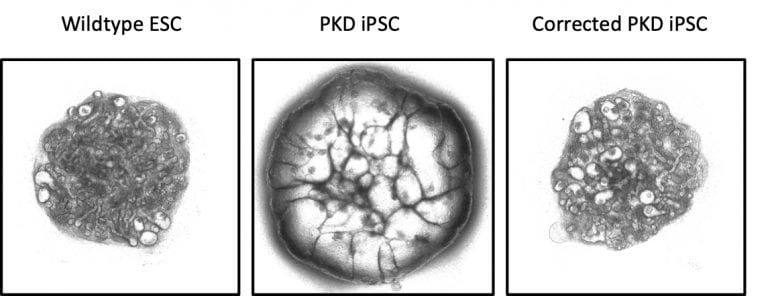 Figure 3: 3D kidney organoids derived from (left) embryonic stem cells; (centre) induced pluripotent stem cells derived from patients with inherited polycystic kidney disease; and (right) gene-corrected induced pluripotent stem cells from patients with inherited polycystic kidney disease. Credit: Cell
Stem Cell (2019), DOI: 10.1016/j.stem.2019.06.009, reproduced with permission.
Figure 3: 3D kidney organoids derived from (left) embryonic stem cells; (centre) induced pluripotent stem cells derived from patients with inherited polycystic kidney disease; and (right) gene-corrected induced pluripotent stem cells from patients with inherited polycystic kidney disease. Credit: Cell
Stem Cell (2019), DOI: 10.1016/j.stem.2019.06.009, reproduced with permission.
Using the organoid-based in vitro model for polycystic kidney disease, we discovered the ability of two chemical compounds to effectively inhibit cyst growth in a dose-dependent manner. We are currently collaborating with the Duke-National University of Singapore Medical School to generate kidney organoids with phenotypes typically seen in diabetic nephropathy—a type of kidney damage resulting from diabetes. Working with analytical chemist Asst Prof Fang Mingliang of NTU’s School of Civil and Environmental Engineering, we are also investigating metabolic reprogramming that occurs during the progression of kidney disease, with the aim of establishing a platform to perform drug screening.


.tmb-listing.jpg?Culture=en&sfvrsn=ab6472c8_1)

.tmb-listing.jpg?Culture=en&sfvrsn=8fcd2938_1)
.tmb-listing.jpg?Culture=en&sfvrsn=a0428bd8_1)
.tmb-listing.jpg?Culture=en&sfvrsn=7a33f2b7_1)