Post-Approval Monitoring
Introduction
Under Singapore’s National Advisory Committee for Laboratory Animal Research (NACLAR)'s Guidelines, NTU-IACUC has the responsibility to ensure the conduct of animal procedures are in compliance with approved Animal Use Protocols (AUPs). This is achieved by conducting regular Post-Approval Monitoring (PAM) .
The PAM Framework in NTU consists of :
- Checks on research animals by NTU ARF husbandry staff and ARF veterinarians.
- Annual Review of all active approved AUPs by NTU IACUC. (Refer to section C in the link for more details)
- Scheduled PAM Visits by IACUC PAM Monitors and IACUC Attending Veterinarian.
Scheduled PAM Visit
Scheduled PAM Visit was established as part of NTU-IACUC's effort to monitor all active NTU-IACUC approved AUPs conducted at NTU and to partner with Principal Investigators and study team members to improve and refine AUP procedures.
The key objectives of the PAM Visit are to:
- Evaluate the understanding and knowledge of NTU animal users to ensure that animal use adhere to applicable ethical and welfare standards, laws, regulations, rules, guidelines, and policies.
- Review research activities to ensure NTU animal users' compliance with approved AUPs and NTU policies.
PAM Visit Process Workflow
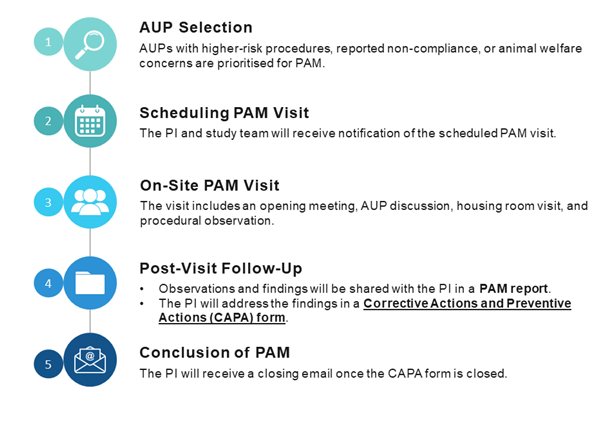 Scope of Review
Scope of Review
- Personnel Competency
- Compliance with Approved AUP
- Anaesthesia / Neuromuscular Blocking Agents
- Surgery / Procedure
- Post-Surgical / Procedural Care
- Euthanasia
- Management of Controlled Drugs
- Study-related Incidents and Safety Monitoring
- Animal Numbers And Housing
Personnel Involved in PAM Visit
- Principal Investigator and Key Study Team Member(s)
- NTU-IACUC Attending Veterinarian
- NTU ARF Veterinarian
- NTU-IACUC PAM Monitors

