Review Not Required (RNR) Category
If your research does not involve (1) any intervention or interaction with human subjects, and (2) does not involve the use of identifiable data or identifiable human biological materials (HBM), then your research is NOT considered “Human subject research (HSR)” and hence approval from IRB is NOT required.
The RNR category is to facilitate researchers conducting secondary research on non-identifiable datasets/HBM that has no potential to cause harm to human subjects.
To meet the criteria for the RNR Category, the following are required:
- There will be strictly NO intervention or interaction with the subjects. Any attempt to re-contact the subjects to collect further data or HBM is prohibited.
- There will be strictly NO usage or access to any identifiable data or identifiable HBM. Any attempt to re-identify the data or HBM (from the original source) is prohibited, and could be a breach of local legislations.
- The research is NOT under the scope of the Human Biomedical Research Act (HBRA), nor Restricted or Prohibited Human Biomedical Research (HBR). Such studies will still require IRB review.
Notes:
a) If the study team initially have access to identifiable data or identifiable HBM, but have then recorded the data in an anonymised form, this does NOT meet the criteria for RNR as the data is considered potentially re-identifiable. Such studies should be submitted to IRB under Exempt Category 4.
b) If the study team is involved in any human tissue banking activities, even if the Human Tissues have been anonymised, such research activities may require a HTF Declaration/Notification to NTU even though it may fall under the scope of RNR.
Process:
PIs whose research fall under the scope of RNR category may submit the RNR Declaration Form on ERMP. These will not be reviewed by the IRB but will be screened by the IRB Secretariat. An acknowledgement letter will be generated after being successfully screened.
Benefits of obtaining an RNR acknowledgement from the IRB:
- To show proof (e.g. to journals) that your research project has been assessed as not requiring IRB review and approval.
- Should your research be deemed not fulfilling the criteria of RNR, and requires IRB approval, note that retrospective approvals by the IRB is not possible once the research has already started.
Example Scenarios
| Fulfils RNR | Does NOT fulfil RNR (Requires IRB Review) |
|---|---|
| 1. NTU study team receives the anonymised or de-identified data/ HBM from collaborator outside of NTU (e.g. NUS, Imperial College, TTSH). Exception: If a member of your team was involved in the original study (e.g. involved in collection of data/HBM, or in data analysis), from which you will be obtaining the data from, then this study requires IRB review and approval as the data is considered potentially re-identifiable. [Refer to Decision Tree for which review category to apply under.] 2. A Trusted Third Party (TTP) is engaged in extracting or providing the data/ HBM in anonymised form. | 1. NTU study team obtains or have access to identifiable data (including publicly available data e.g. social media) but anonymises the data for research. [=> Exempt Category 4] 2. NTU study team extracts data / HBM from their own past research projects in anonymised form such that the data can no longer be linked to the individuals. [=> Exempt Category 4] |
For further guidance on which category to apply for, please refer to our Decision Tree.
FAQ
Q1. My study was previously approved under the Exempt Category 4 but seems to fit into the new RNR category. Should I submit the RNR form now? |
Q2. Is it mandatory to apply for RNR if my study fits the RNR criteria? |
Q3. My study conducts secondary analysis of anonymised data/ HBM but also involves interviews/ interventions on human participants. Can I apply for RNR? |
Q4. How do I submit an RNR application? |
RNR Application Guide
Step 1: Create a New Application in ERMP
Step 2: Select ‘Review Not Required (RNR)’ under Review Category
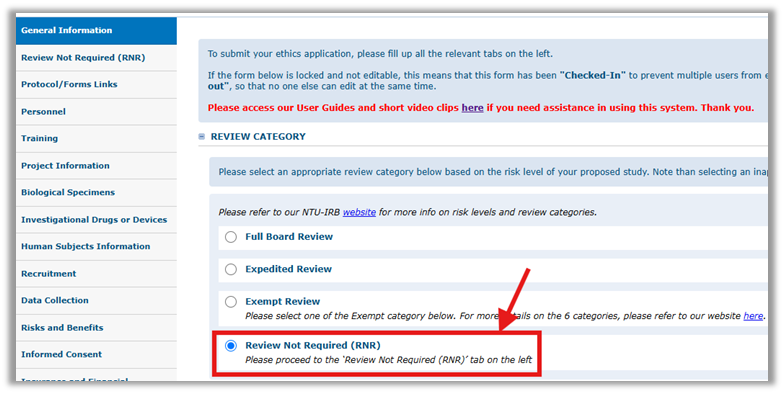
Step 3: Complete the ‘Review Not Required (RNR)’ tab
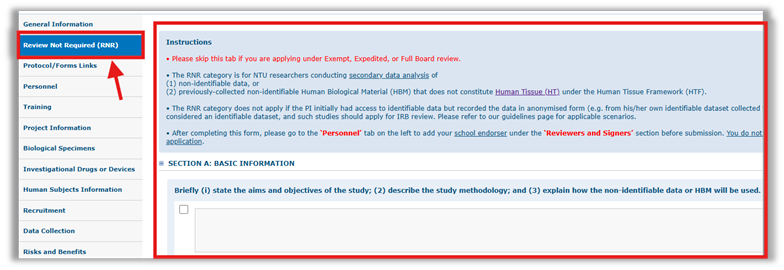
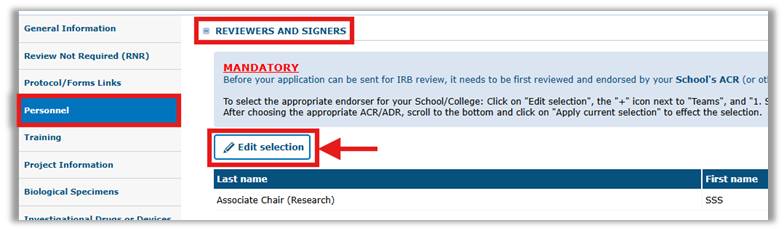
Step 4: Add your School Endorser under the ‘Reviewers and Signers’ section in the ‘Personnel Tab’
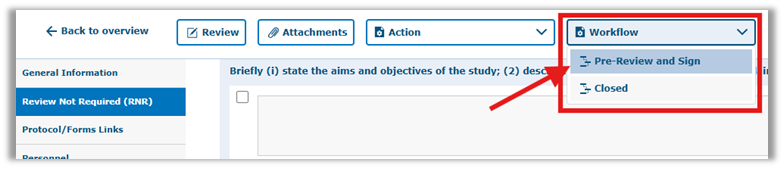
Step 5: Click ‘Pre-Review and Sign’ in the ‘Workflow’ dropdown list to submit your application.
Step 6: You will be notified once approved and receive an RNR letter attached to your ERMP application.

